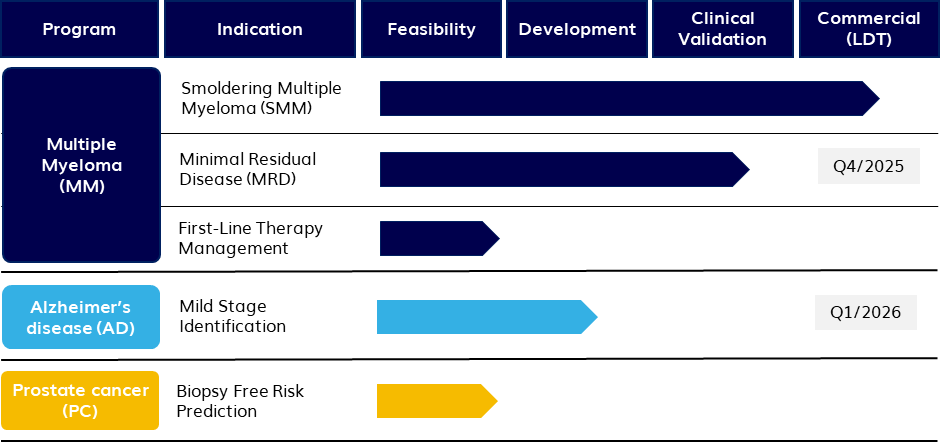
Smoldering Multiple Myeloma (SMM) :TeloViewSMM has been developed in collaboration with Mayo Clinic, commercial rights are owned by Telo. This assay identifies a patient's risk of progression from the precursor Smoldering Multiple Myeloma to incurable Multiple Myeloma.
TeloViewSMM is in a Real-World Experience Study with select clinical centers and also commercially available as an LDT.
Minimal Residual Disease (MRD): TeloMRD for multiple myeloma is in a clinical trial in collaboration with Cleveland Clinic. It is the first clinical TeloView assay designed to work on a liquid biopsy of filter captured blood cells. The trial is expected to lead to a commercially available MRD product in Q4 2025 and a risk scoring algorithm that can be combined with MRD counting in Q2 2026.
First-line therapy management:TeloViewMM is in a clinical trial in collaboration with Mayo Clinic. A risk scoring algorithm has been trained in an initial cohort which can identify patients' risk of relapse with standard first line treatment. A second cohort is being processed to blindly validate the predictive value.
Mild Alzheimer’s disease identification:TeloViewAD is designed to work with a cheek swab sample. TeloView parameters that can identify AD with high sensitivity and specificity from cheek cells have been identified in previous studies. A predictive clinical algorithm is being developed is in collaboration with Sunnybrook Hospital.
Biopsy Free Risk Prediction:TeloViewPC stratifies intermediate risk (gleason score 7) Prostate Cancer patients into categories of aggressive vs. benign disease and avoids unnecessary biopsy and surgery. The assay has a preliminary predictive risk algorithm ready and Telo is seeking partners to validate it clinically.